All information collected during this clinical study is kept strictly confidential in accordance with IRB guidelines and federal regulations (HIPAA). Your personal identifying information will not be shared without your explicit consent. Study data is de-identified and used only for research purposes.

About the Clinical Study
UNITED is a U.S. FDA-supervised clinical study evaluating an investigational penile prosthesis. The study is collecting information on the safety and performance/effectiveness of the Rigicon Infla10® Pulse™ Dynamic Inflatable Penile Prosthesis in men with erectile dysfunction.

UNITED is a U.S. FDA-supervised clinical study evaluating an investigational penile prosthesis. The study is collecting information on the safety and performance/effectiveness of the Rigicon Infla10® Pulse™ Dynamic Inflatable Penile Prosthesis in men with erectile dysfunction.
What Is Being Studied?
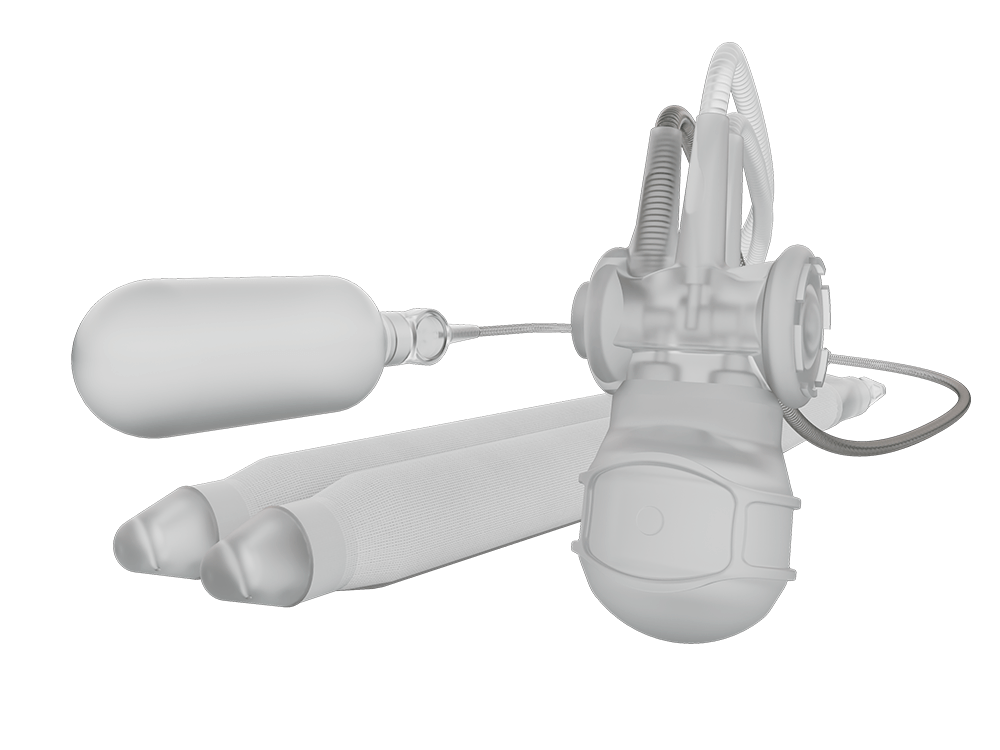
The device under evaluation is the Infla10® Pulse™ DIPP, an investigational three-piece inflatable penile prosthesis designed to allow manual control of penile rigidity. Because it is investigational, it is not currently FDA-approved for commercial use in the United States.
About Infla10® Pulse™ Dynamic Inflatable Penile Prosthesis
The device is an investigational product being evaluated in the United Clinical Study for Infla10® Pulse™ DIPP. It is intended for men who may be candidates for penile prosthesis implantation. This three-piece, fluid-filled system (two cylinders, pump, reservoir) is designed to allow the user to manually control penile rigidity.
Global Experience & U.S. Status: While the Infla10® is currently classified as an investigational device in the United States, it is commercially available and implanted in over 40 countries worldwide—including the United Kingdom, European Union, Australia, and South Korea.
The UNITED Clinical Study is being conducted to collect data specifically to evaluate the safety and effectiveness of this device for patients in the United States. Participation in this study offers eligible men the opportunity to receive this technology as part of a regulated clinical trial.
Important Regulatory Information: This device is investigational and is being evaluated for safety and effectiveness in clinical studies. Bench testing data are on file; however, no clinical performance conclusions can be drawn from bench data, and such testing is not predictive of individual clinical outcomes.
Individual outcomes may vary based on anatomical factors and surgical technique. Caution: Investigational device. Limited by Federal (or United States) law to investigational use.
What Does Participation Involve?
Participation is voluntary and involves a structured follow-up period of approximately 36 months after the device is implanted. During this time, the study team will monitor the device's performance and your safety.
- Screening: A medical evaluation to determine if you qualify.
- Procedure: Surgical implantation of the investigational device.
- Follow-Up: Scheduled clinic visits at regular intervals (e.g., post-op, 3, 6, 12, 18, 24, and 36 months) to assess your recovery and device function.
Financial Information & Insurance
Medicare & Insurance Coverage (Category B IDE)
The UNITED Study is classified by CMS as a Category B IDE study. This means that for eligible beneficiaries (including Medicare), routine care services, such as the surgery, hospital stay, and standard doctor visits, may be covered by your insurance or Medicare, subject to your plan's deductibles and co-pays.
Patient Responsibility: If your insurance does not cover the standard costs associated with your medical care, you will be responsible for those expenses. Your study team will review any potential out‑of‑pocket costs with you before you decide whether to participate.
Reimbursement: Participants will be reimbursed for study‑related expenses such as travel or parking. Your study team will review your reimbursement with you.
Potential Benefits and Risks
Transparency is key to our research. Here is what you should consider.
Potential Benefits
Because this is an investigational clinical study, there is no guarantee that you will receive a direct medical benefit from participation. However, the Infla10® Pulse™ IPP is designed with the following intended purposes:
- Intended to Support Erectile Function: The device is designed with the intent to support erectile function by allowing the user to manually control penile rigidity, which may help some patients engage in sexual activity.
- User-Controlled Design: The three-piece inflatable system is designed to allow the user to control when the penis is rigid and when it is flaccid, based on personal needs and daily activities.
- Contribution to Medical Research: By participating in this clinical study, you provide valuable data that may help researchers evaluate the safety and effectiveness of this investigational device for future patients in the United States.
Potential Risks
Participation in this clinical study involves a surgical procedure and the use of an investigational medical device. As with any surgery or investigational treatment, there are potential risks.
These may include:
- Risks related to surgery or anesthesia
- Risks associated with the device itself
- The possibility of complications that are not yet known
The study doctor will review all known and potential risks with you in detail during the informed consent process before you decide whether to participate. Participation is voluntary, and you may choose not to participate or to withdraw at any time.
Am I Eligible?
Eligibility for this clinical study is determined by specific medical criteria. Your healthcare provider will review these criteria with you during the screening process. Participation is voluntary and eligibility is determined by the study team. Not all individuals will qualify.
General Eligibility Considerations
- Diagnosis of erectile dysfunction
- May be a candidate for penile prosthesis implantation (per investigator judgment)
- Ability to attend all scheduled follow-up visits
- Willingness to complete study questionnaires
- No medical conditions that would contraindicate surgery
Important Note
Final eligibility is determined by the study team through a comprehensive medical evaluation. Completing the online ED self-check does not guarantee study eligibility or enrollment.
Your Privacy is Protected
Your privacy and confidentiality are our top priorities throughout the clinical study process.
Important Privacy Notice
Do not submit personal health information through this website outside designated study forms. For medical questions or concerns, please contact the study team directly.
Have Questions?
If you have questions about the clinical study, eligibility criteria, or what participation involves, please contact us or speak with your urologist.
Contacting a site does not imply eligibility or enrollment.
Medical Emergencies
Important Note: Do not send medical emergencies via this site; call 911.
Study Registration
ClinicalTrials.gov ID: NCT07273773